Consortium
- Project Management Juelich - Germany
- Bayer AG - Germany
- Christian-Albrechts-University Kiel - Germany
- Erasmus Medical Center Rottedam - The Netherlands
- European Molecular Biology Laboratory/ELIXIR - United Kingdom
- HITS gGmbH - Germany
- Federal Agency for Medicines and Health Products - Belgium
- German Institute for Standardization - Germany
- Karolinska Institutet - Sweden
- Qiagen - Germany
- University College London - United Kingdom
- University of Copenhagen (Faculty of Law) - Denmark
- University of Copenhagen (Disease Systems Biology) - Denmark
- University of Oxford - United Kingdom
- University of Parma - Italy
- University of Rostock - Germany
- Vilnius University - Lithuania
Strategic Objectives
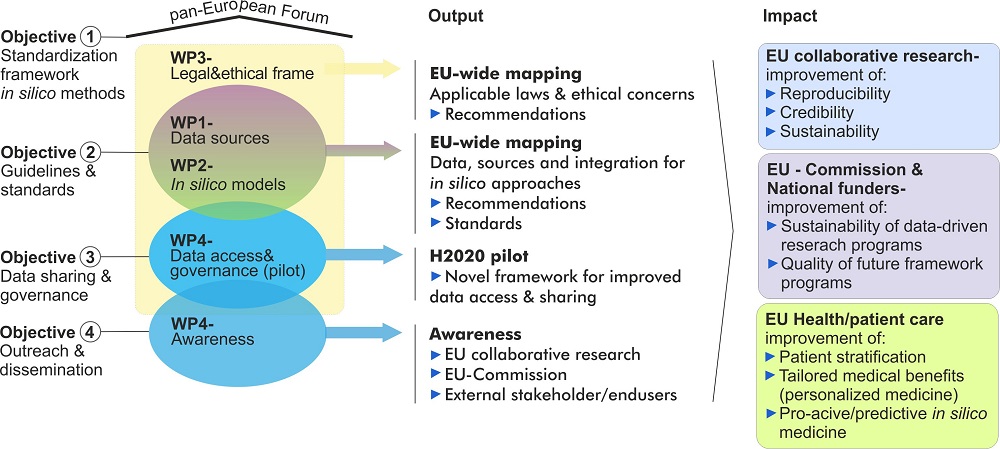
To develop relevant standards for in silico approaches in personalised medicine EU-STANDS4PM will be implemented as a pan-European expert forum and framework that has the following strategic objectives:
To establish a pan-European standardization framework for in silico methodologies applied in personalised medicine
We will assemble and implement EU-STANDS4PM as a framework composed of cutting edge European research projects, clinical, legal and ethical experts as well as regulatory bodies, industry and standardization organisations. This Forum will represent state-of-the-art expertise with regard to:
- Data sources and standards for predictions in personalised medicine
- Integrated data analysis and data-driven in silico models in personalised medicine
- Relationships between law, ethics and the emerging technologies in health
- Industry and regulatory needs for standards in personalised medicine
- Implementation of European-level and international standards
A central task of EU-STANDS4PM, its key experts and complemented expertise will be to evaluate (trans)national standardization strategies for health data integration as well as data-driven in silico modelling approaches for personalised medicine within Europe. For this purpose EU-STANDS4PM comprises a core of five ongoing H2020 collaborative projects (BD2Decide, SysCID, LifeCycle, MultipleMS and SPIDIA4P) that cover the essential aspects of this objective and will be linked to further transnational initiatives.
To develop pan-European, generally admitted recommendations for standardization guidelines for in silico methodologies applied in personalised medicine
To exploit the full potential of Big Data in health research EU-STANDS4PM will make an Europe-wide assessment which data sources are best suitable for data harmonisation as well as integration approaches that can, in the medium and long term, lead to the interpretation of health data through innovative in silico approaches in clinical research and practice across Europe. Based on this broad and community overarching assessment, EU-STANDS4PM will establish a catalogue of current practice as well as define recommendation for standardized interoperable approaches and data integration strategies that allow the relevant research communities (e.g. current and future H2020 collaborative research projects) for the development of in silico models to enable personalised medicine.
To ensure highest visibility, credibility and authority of the EU-STANDS4PM output, we will contribute to the drafting of international standards, especially in already established ISO (the International Organization for Standardization) working groups within the ISO technical committee. Through partner German Institute for Standardization (DIN) we also aim at establishing a new working group within the European Technical Committee with the goal to initiate the development of standard documents on a European level (European Committee for Standardization, CEN) on data integration strategies and in silico models to be used in clinical research/practice.
To improve data sharing, dissemination and governance for European collaborative research projects
In close collaboration with Global Alliance for Genomics and Health (GA4GH) and other established standardization initiatives, we will develop an innovative strategy for improved data sharing, dissemination and governance for European collaborative research projects focussing on large scale health data to efforts to ensure that (i) the needs of European projects are visible and (ii) conversely that emerging EU projects are aware and can easily apply internationally accepted standards. The core of our five participating H2020 projects (see above) will guide this process and we will analyse their status quo, hurdles, bottlenecks, and expectations but also best practices, optimisation strategies and success stories. We will use these projects as pilot to reach out to other external projects dealing with similar issues.
To reach out and maximize impact of EU-STANDS4PM´s recommendations in research, national funding schemes and policy
To ensure highest visibility of EU-STANDS4PM´s work, as well as to secure an active involvement and feedback of key stakeholders, we will follow a pro-active and structured strategy for project’s outreach and organise a series of specific awareness events targeting the following groups:
- Collaborative research and networking projects from the mentioned funding programs
- EU funding organisations (e.g. funders active in ICPerMed)
- Regulatory bodies (e.g. European Medicines Agency)
- Patient organisations (e.g. European Patient´s Forum)
To enable a widespread use of best practices in European collaborative research projects, we will disseminate, through the above channels, the EU-STANDS4PM´s output and maximize impact on these communities. We will have a specific focus on national funding organisations, including the European Commission. The aim is to include EU-STANDS4PM´s recommendations into future calls for collaborative research projects. In addition we will establish a communication channel to regulatory bodies to harmonise EU-STANDS4PM´s work with regard to standards already in use by these authorities.
A key output of the project will be recommendations for standards and best practices for data harmonisation/integration strategies as well as data-driven in silico approaches to interpret human disease/health data.
EU-STANDS4PM will create a consciousness among its extended European stakeholder network for key standardization determinants in order to enable a truly in silico driven personalised medicine in the medium and long term in clinical research and practice.
Work plan structure
A major aim of the project is to set up a European framework that will be able to implement the specific objectives of the project. For this purpose EU-STANDS4PM is composed of five work packages that complement each other as described in the overview below.
Objectives
- Develop an overview of existing and emerging European and international standards relevant to the integration of personalised medicine data, e.g. standard formats, minimal information guidelines and metadata standards (i.e. ontologies and controlled vocabularies), data access protocols and ELSI protocols to allow cross-border data access and integration.
- Drive the further harmonization and interoperability of domain-specific standards in the different life science domains contributing to the aims of personalised medicine (both, scientific “grass-roots” community standards, and standards set by standard setting bodies), in order to facilitate data integration, model building and prediction.
- Consult outcomes with the larger community to produce an action plan to secure broad, long-term access of readily integratable data resources for personalised medicine to facilitate review and alignment of in silico modelling strategies.
Objectives
- Review data integration approaches and in silico models of relevance for translational research that address longitudinal analysis of health data
- Define best practices for in silico models for transnational personalised medicine efforts
- Define and outline standards for longitudinal data of relevance for comparative analysis of disease trajectories across European countries
Objectives
- Map and analyse European and international data protection and clinical trials regulation in the area of data protection, biomedical research (including clinical trials), and patients’ rights relevant for collaborative research for heterogeneous data integration and data driven in silico models.
- Evaluate legal and ethical challenges and explore possibilities for collaborative research for heterogeneous data integration and data driven in silico models
- In collaboration with the other WP’s and external stakeholders: Develop recommendations which are compliant with the current legal and ethical framework and provide a sustainable backbone for collaborative research for heterogeneous data integration and data driven in silico models.
Objectives
- Implement a progressive dissemination to initiate and implement three innovative objectives for improved data sharing, dissemination and governance for future collaborative EU research projects with focus on:
(a) Development and implementation of a harmonised Data Access Agreement (hDAA);
(b) Implementation of Registered Access (RA) and
(c) Evaluation of cloud-based access (CbA) to EU project data - Set up a pro-active and structured outreach strategy to assure awareness of the EU-STANDS4PM output to national funding organisations, EU-Commission, regulatory authorities as well as patient organisations, and the general systems/personalised medicine community.
Objectives
- Establishing and maintaining an efficient administrative infrastructure within the consortium to ensure a streamlined day-to-day operational procedure process.
- Implementation and supervising of an efficient management and decision-making procedure ensuring adequate internal and external communication and appropriate flow of information.
- Establishing a transparent communication between consortium partners, the EU-Commission and all relevant external stakeholders.
- Managing the consortium budget and all official EU-Commission reporting duties.